NXR Cryosperm IVF (Straws)
(LN2)
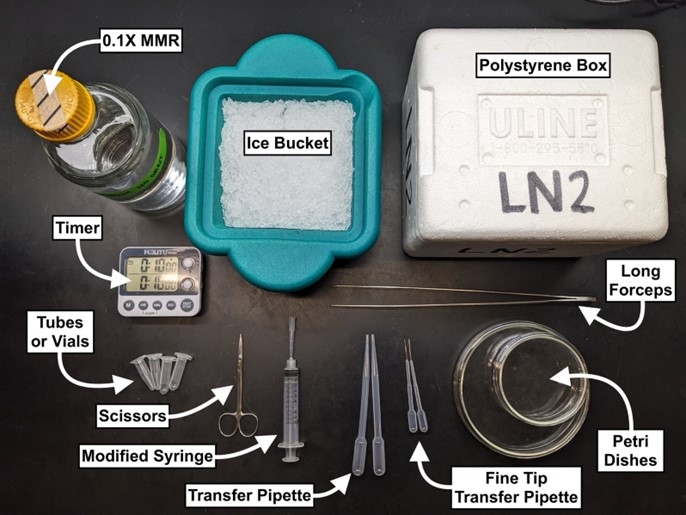
Prepare a station (near a water bath) with each of the following:
- Water bath (set to 37-40°C)
- Long stainless steel forceps (8”+ length)
- Ice bucket
- Scissors
- 10mL syringe with inverted 200uL tip inserted (see image below)
- Transfer pipettes (General Purpose, Samco #202-20S)
- Transfer pipettes (Extended Fine Tip, Small Bulb, Samco #231)
- 1mL+ tubes
- Glass or plastic Petri dishes (size dependent on use)
- 0.1x MMR
- Timer
1. Fill a storage container with enough LN2 to submerge straw samples.
2. Collect straws from storage (vapor shipping dewar, storage dewar) and immediately submerge in LN2 filled container.
Chill tips of forceps in the LN2 to allow them to cool before bringing in contact with samples (~3-5s). Move samples between storage securely but quickly to limit exposure to air temperature.
3. Manipulate straw samples with long forceps in the LN2 and check the label to confirm correct samples.
Avoid removing the tubes from LN2 until ready to thaw.
4. Fill a container with ice and insert one tube per straw sample to be thawed.
Samples do not require addition of 0.1x MMR to result in successful fertilizations. Adding 0.1x MMR (~50-250μL) to thawed samples increases the volume that can be spread across eggs.
5. Squeeze eggs from hormonally induced X. laevis onto a prepared Petri dish.
One straw can fertilize 400+ eggs.
6. Remove excess liquid from the dish by dumping (if eggs are sticking to the plate) or with a transfer pipette.
If storing eggs in MMR (1x or similar) make sure to remove all MMR before applying the sample.
7. If using 0.1x MMR to extend sample volume, fill a pipette and set aside.
8. Using long forceps, gather a straw from the LN2 container and quickly transfer it to a 37- 40°C water bath. Move the straw back and forth in the water bath for 5 seconds to ensure the sample thaws evenly.
9. Remove straw from the water bath and dry the exterior of the straw with a paper towel or wipe.
Hold the straw from the end with the cotton plug to avoid excessively warming the sample due to contact.
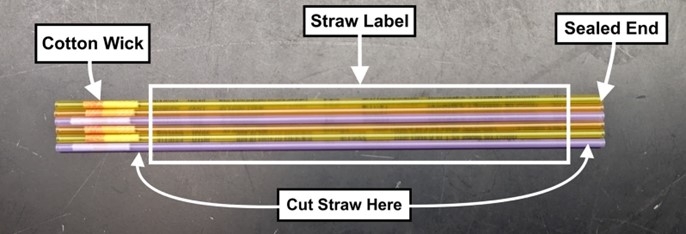
10. Hold the straw over a waste container and snip off the sealed end.
Cut as close to the end of the straw as possible to avoid losing sample.
11. Move the open end of the straw into an empty tube on ice and cut the other end just below the cotton wick to release the sample.
Most of the sample will fall into the open tube when the straw is cut. Any remaining sample can be pushed into the tube with the modified syringe, depressing the plunger to push out any remaining volume.
12. Pipette the sample from the tube and apply across all areas of eggs on a Petri dish.
If extending sample volume, add 0.1x MMR to the tube prior to applying.
13. Using the other transfer pipette (Extended Fine Tip) mix and spread eggs across the Petri dish into a monolayer to ensure all are exposed to the sample.
Leaving eggs in clumps can result in poor fertilization and reduced survival of embryos.
14. Incubate embryos at room temperature (~21°C) for 10 minutes.
15. After 10 minutes, fill the dish with enough 0.1x MMR to submerge the eggs.
16. Incubate embryos at desired temperature.
(Vapor Shipper)
Prepare a station (near a water bath) with each of the following:
- Water bath (set to 37-40°C)
- Long stainless steel forceps (8”+ length)
- Ice bucket
- Scissors
- 10mL syringe (no needle) with inverted 200uL tip inserted (see below)
- Transfer pipettes (General Purpose, Samco #202-20S)
- Transfer pipettes (Extended Fine Tip, Small Bulb, Samco #231)
- 1mL+ tubes
- Glass or plastic Petri dishes (size dependent on use)
- 0.1x MMR
- Timer
1. Move the vapor shipper near a water bath.
2. Fill a container with ice and insert one tube per straw sample to be thawed.
Samples do not require addition of 0.1x MMR to result in successful fertilizations. Adding 0.1x MMR (~50-250μL) to thawed samples increases the volume that can be spread across eggs.
3. Squeeze eggs from hormonally induced X. laevis onto a prepared Petri dish.
One straw can fertilize 400+ eggs.
4. Remove excess liquid from the dish by dumping (if eggs are sticking to the plate) or with a transfer pipette.
If storing eggs in MMR (1x or similar) make sure to remove all the MMR before applying the sample.
5. If using 0.1x MMR to extend sample volume, fill a pipette and set aside.
6. Lift the lid of the vapor shipper so the hanging basket sits just below the neck of the shipper and visualize samples.
Chill tips of forceps in the neck of the vapor shipper to allow them to cool before bringing in contact with samples (~5-8s). Move samples between storage securely but quickly to limit exposure to air temperature.
7. Using long forceps, gather a straw from the vapor shipper and quickly transfer it to a 37-40°C water bath. Move the straw back and forth in the water bath for 5 seconds to ensure the sample thaws evenly.
8. Remove straw from the water bath and dry the exterior of the straw with a paper towel or wipe.
Hold the straw from the end with the cotton plug to avoid excessively warming the sample due to contact.
9. Hold the straw over a waste container and snip off the sealed end.
Cut as close to the end of the straw as possible to avoid losing sample.
10. Move the open end of the straw into an empty tube on ice and cut the other end just below the cotton wick to release the sample.
Most of the sample will fall into the open tube when the straw is cut. Any remaining sample can be pushed into the tube with the modified syringe, depressing the plunger to push out any remaining volume.
11. Pipette sample from the tube and apply across all areas of eggs on a Petri dish.
If extending sample volume, add 0.1x MMR to the tube prior to applying.
12. Using the other transfer pipette (Extended Fine Tip) mix and spread eggs across the Petri dish into a monolayer to ensure all are exposed to the sample.
Leaving eggs in clumps can result in poor fertilization and reduced survival of embryos.
13. Incubate embryos at room temperature (~21°C) for 10 minutes.
14. After 10 minutes, fill the dish with enough 0.1x MMR to submerge the eggs.
15. Incubate embryos at desired temperature.