NXR Cryosperm IVF (Tubes)
(Dry Ice)
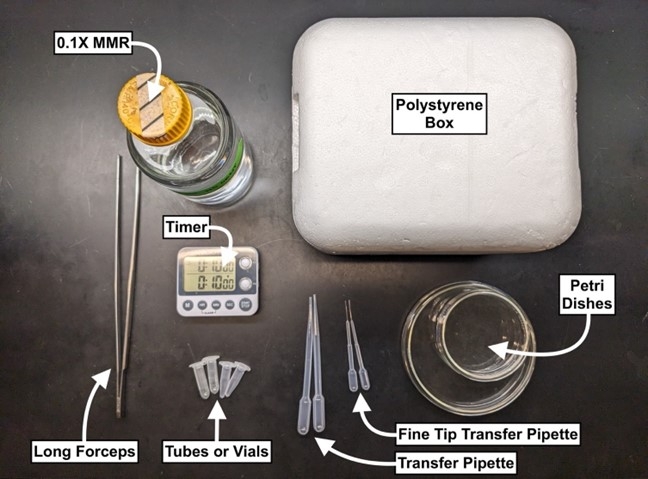
Prepare a station (near a water bath) with each of the following:
- Water bath (set to 37-40°C)
- Polystyrene box (filled with dry ice)
- Long stainless steel forceps (8”+ length)
- Transfer pipettes (General Purpose, Samco #202-20S)
- Transfer pipettes (Extended Fine Tip, Small Bulb, Samco #231)
- 1mL+ tubes
- Glass or plastic Petri dishes (size dependent on use)
- 0.1x MMR
- Timer
1. Fill a polystyrene box with enough dry ice to hold 0.5mL tubes.
2. Collect tube(s) from storage (vapor shipping dewar, dry ice shipping box, storage dewar, -80°C freezer) and place in dry ice.
Hold tube only by cap or using chilled forceps to avoid heating the sample before thawing.
3. Prepare a tube with 0.1x MMR to extend thawed sample volume.
Samples do not require addition of 0.1x MMR to result in successful fertilizations. Adding 0.1x MMR (~50-150μL) to thawed samples increases the volume that can be spread across eggs.
4. Squeeze eggs from hormonally induced X. laevis onto a prepared Petri dish.
One tube can fertilize 400+ eggs.
5. Remove excess liquid from the dish by dumping (if eggs are sticking to the plate) or with a transfer pipette.
If storing eggs in MMR (1x or similar) make sure to remove all MMR before applying the sample.
6. If using 0.1x MMR to extend sample volume, fill a pipette and set aside.
7. Using long forceps, or by hand, gather a tube from the dry ice and quickly transfer to a 37-40°C water bath. Constantly move the tube in the water bath for ~33s to ensure the sample thaws evenly.
Any remaining chunk of frozen sample can be thawed with an extra 1-2s in the water bath or will finish thawing upon addition of 0.1x MMR.
8. Remove sample from water bath and uncap tube.
If extending sample volume, add 0.1x MMR to the tube.
9. Pipette sample from the tube and apply across all areas of eggs on a Petri dish.
10. Using the other transfer pipette (Extended Fine Tip) mix and spread eggs across the Petri dish into a monolayer to ensure all are exposed to the sample.
Leaving eggs in clumps can result in poor fertilization and reduced survival of embryos.
11. Incubate embryos at room temperature (~21°C) for 10 minutes.
12. After 10 minutes, fill the dish with enough 0.1x MMR to submerge the eggs.
13. Incubate embryos at desired temperature.
(LN2)
Prepare a station (near a water bath) with each of the following:
- Water bath (set to 37-40°C)
- Polystyrene box or other LN2 safe container
- Long stainless steel forceps (8”+ length)
- Transfer pipettes (General Purpose, Samco #202-20S)
- Transfer pipettes (Extended Fine Tip, Small Bulb, Samco #231)
- 1mL+ tubes
- Glass or plastic Petri dishes (size dependent on use)
- 0.1x MMR
- Timer
1. Fill a LN2 storage container with enough LN2 to float sample tubes.
Tubes may float or sink in the LN2.
2. Collect tube(s) from storage (vapor shipping dewar, dry ice shipping box, storage dewar, -80°C freezer) and immediately place in the filled LN2 storage container.
Hold tube only by cap or using chilled forceps to avoid heating the sample before thawing.
3. Manipulate tubes with forceps in the LN2 and check the label to confirm correct samples.
Avoid removing the tubes from LN2 until ready to thaw.
4. Prepare a tube with 0.1x MMR to extend thawed sample volume.
Sample does not require addition of 0.1x MMR to result in a successful fertilization. Adding ~50-150μL to thawed samples increases volume that can be spread across eggs.
5. Squeeze eggs from hormonally induced X. laevis onto a prepared Petri dish.
One tube can fertilize 400+ eggs.
6. Remove excess liquid from the dish by dumping (if eggs are sticking to the plate) or with a transfer pipette.
If storing eggs in MMR (1x or similar) make sure to remove all the MMR before applying the sample.
7. If using 0.1x MMR to extend sample volume, fill a pipette and set aside.
8. Using long forceps, gather a tube from the LN2 and quickly transfer to a 37-40°C water bath. Constantly move the tube in the water bath for ~33s to ensure the sample thaws evenly.
Any remaining chunk of frozen sample can be thawed with an extra 1-2s in the water bath or will finish thawing upon addition of 0.1x MMR.
9. Remove sample from water bath and uncap tube.
If extending sample volume, add 0.1x MMR to tube.
10. Pipette sample from the tube and apply directly to all areas of eggs on a Petri dish.
11. Using the other transfer pipette (Extended Fine Tip) mix and spread eggs across the Petri dish into a monolayer to ensure all eggs are exposed to the sample.
Leaving eggs in clumps can result in poor fertilization and reduced survival of embryos.
12. Incubate embryos at room temperature (~21°C) for 10 minutes.
13. After 10 minutes fill the dish with 0.1x MMR to fully cover the eggs.
14. Incubate embryos at desired temperature.