MBL Study Details Impact of Genetic Defect on Nerve Cells in Parkinson's Disease
Contact: Diana Kenney, Marine Biological Laboratory 508-289-7139; dkenney@mbl.edu
Parkinson’s disease is a slow-progressing neurodegenerative disorder that afflicts about 4 to 6 million people worldwide, most of them over the age of 50. Its major symptoms are problems with movement: shakiness, slowness, rigidity, imbalance, and difficulty walking. There is no known cure. The causes of Parkinson’s are unknown, but some cases are inherited.
To explore how the neurodegeneration in Parkinson’s occurs, MBL Associate Scientist Jennifer Morgan is using the sea lamprey, a vertebrate fish, as a model for the human condition.
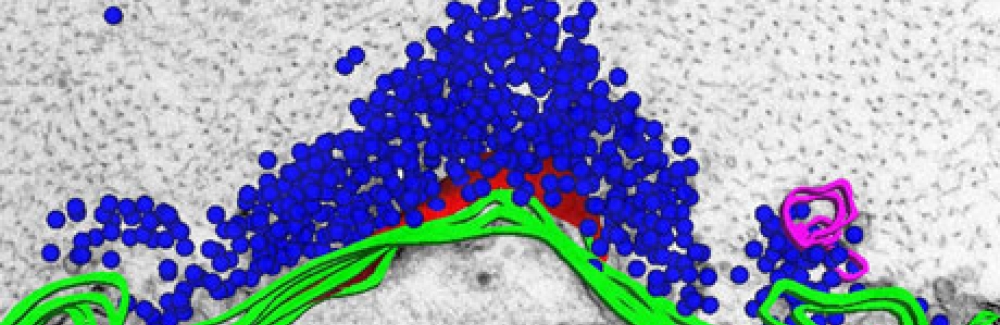
Both lamprey and humans have a set of neurons, called reticulospinal cells, whose axon processes extend from the brain to the spinal cord to control movement. In the lamprey, some of these neurons are giant, compared to human nerve cells. Their giant synapses, where the cell communicates with other nerve cells, have ten times more vesicles sending out neurotransmitter than human synapses do. The size of these lamprey neurons makes them an excellent system for studying basic processes of neural communication and what goes wrong in diseases such as Parkinson’s.
In a recent study, Morgan and her colleagues replicated in the lamprey the problems caused by a genetic defect that leads to Parkinson’s disease. These patients have too many copies of the α-synuclein gene, which leads to an overabundance of the α-synuclein protein and its formation into toxic aggregates throughout the neuron, including at synapses. For reasons that aren’t yet understood, these protein aggregates eventually kill the motor neurons that lead to the disease’s movement disorders.
“We found that when we introduce too much of the synuclein protein to the [lamprey] synapse in levels that mimic what is observed in Parkinson’s disease, it dramatically changes the morphology of the synapse,” Morgan says. “You end up with synapses that have very few synaptic vesicles.”
“We need to understand exactly why that is happening, because if the synapse runs out of vesicles, there is no more neurotransmitter, and the synapse can’t work anymore,” Morgan says. “Our next step is to understand how the synuclein causes that defect and figure out ways to reverse it.”
Citation:
Busch DJ et al (2014) Acute increase of α-synuclein inhibits synaptic vesicle recycling evoked during intense stimulation. MBoC 25: 3926-3941. http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-02-0708
Featured image: Reconstruction of a lamprey reticulospinal synapse overlaid onto an electron micrograph of the same synapse. This image appeared on the cover of the February 1, 2015 issue of Molecular Biology of the Cell. Credit: David J. Busch and Paul A. Oliphint, University of Texas at Austin; Jennifer R. Morgan, MBL.
—###—
The Marine Biological Laboratory (MBL) is dedicated to scientific discovery and improving the human condition through research and education in biology, biomedicine, and environmental science. Founded in Woods Hole, Massachusetts, in 1888, the MBL is a private, nonprofit institution and an affiliate of the University of Chicago.