Scientists Develop Novel Assay to Decode Functional Elements of Genome

Contact: Diana Kenney, Marine Biological Laboratory
dkenney@mbl.edu; 508-685-3525
WOODS HOLE, Mass.--One of the most profound changes in the life of an organism is what Antonio Giraldez calls “embryonic puberty”: the stage when an early embryo stops taking instructions from its mother on how to develop and activates its own genome to kick out those instructions instead. This critical stage, called the maternal-to-zygote transition, happens in all embryos, from sea anemones to humans. Yet how it is regulated in the embryo is not yet known.
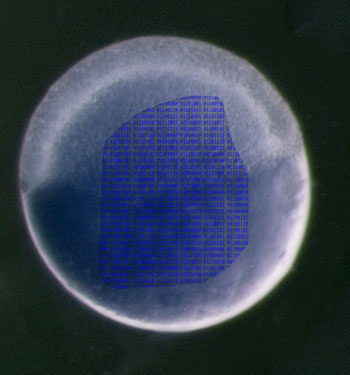 Zebrafish embryo superimposed with a “Rosetta Stone” that reads: "This paper identifies regulatory sequences in RNA by analyzing their regulatory function in a massive parallel reporter assay during embryogenesis. The method is called RESA (RNA Element Selection Assay).” Photo illustration courtesy of Antonio Giraldez
Zebrafish embryo superimposed with a “Rosetta Stone” that reads: "This paper identifies regulatory sequences in RNA by analyzing their regulatory function in a massive parallel reporter assay during embryogenesis. The method is called RESA (RNA Element Selection Assay).” Photo illustration courtesy of Antonio GiraldezThis week in Nature Methods, Giraldez and colleagues present a novel way to decipher the genetic code that embryos use to instruct many maternal messages (mRNAs) to be destroyed, and others to become stabilized. Giraldez is a Professor of Genetics at Yale University School of Medicine and was a 2016 Research Awardee in the MBL Whitman Center, where he conducted part of this research.
The method, called RESA (RNA Element Selection Assay), has broad applications, Giraldez says. “It’s a modular method we can use in many contexts, depending on the question the investigator wants to ask, to dissect the meaning of different parts of the genome. It is a molecular ‘Rosetta Stone’ to help us decode the functional elements within the genome.”
In this case, they used RESA to detect the stability or decay of millions of RNA fragments in the zebrafish embryo, which in turn gave information about the genes that are activated or shut down during the maternal-to-zygote transition.
The team developed RESA in zebrafish, Giraldez says, “but the goal is to use it across many different species, so we can find meaningful ‘words’ or instructions in the genome from squid to mouse to human.” He plans to continue testing RESA in squid and other marine model systems at the MBL next year, such as sea urchin and ctenophores.
“That’s the part I am most excited about, is the MBL offers us this opportunity to test RESA across many species,” Giraldez says. “That is priceless; it’s work that cannot be done anywhere else in the world.”
“The MBL made me realize that we know so much about a few species, and so little about so many other species,” Giraldez says. “But now, with new sequencing technologies like RESA, we can really understand biology much more broadly across species. That is really a new revolution.”
Citation:
Yartseva, Valeria et al (2016) RESA identifies mRNA-regulatory sequences at high resolution. Nature Methods, doi: 10.1038/nmeth.4121
—###—
The Marine Biological Laboratory (MBL) is dedicated to scientific discovery – exploring fundamental biology, understanding marine biodiversity and the environment, and informing the human condition through research and education. Founded in Woods Hole, Massachusetts in 1888, the MBL is a private, nonprofit institution and an affiliate of the University of Chicago.