Microbes in Dental Plaque are More Like Relatives in Soil than on the Tongue
Contact: Diana Kenney dkenney@mbl.edu; 508-605-3525 By Alison Caldwell University of Chicago Medicine
Study suggests plaque may have been a “stepping stone” for microbes into the body
CHICAGO and WOODS HOLE, Mass. -- From the perspective of A. Murat Eren, the mouth is the perfect place to study microbial communities.
“Not only is it the beginning of the GI tract, but it’s also a very special and small environment that’s microbially diverse enough that we can really start to answer interesting questions about microbiomes and their evolution,” said Eren, an assistant professor in the Department of Medicine at the University of Chicago and an MBL Fellow at the UChicago-affiliated Marine Biological Laboratory (MBL), Woods Hole.
“There’s a surprising amount of site specificity, in that you find defined patterns of microbes in different areas of the mouth — the microbes associated with the tongue are very different from those on the plaque on your teeth,” he continued. “Your tongue microbes are more similar to those living on someone else’s tongue than they are to those living in your throat or on your gums!”
 Alon Shaiber of Cornell Weill Medicine, left, and A. Murat Eren (Meren) of University of Chicago and the MBL. Photo courtesy of Meren.
Alon Shaiber of Cornell Weill Medicine, left, and A. Murat Eren (Meren) of University of Chicago and the MBL. Photo courtesy of Meren.In a pair of papers published on Dec. 16 in Genome Biology, Eren, who goes by Meren, and colleagues, including senior co-author and MBL Associate Scientist Jessica Mark Welch, zeroed in on this unique ecology with state-of-the-art sequencing and analysis approaches to get a better picture of the oral microbiome.
Their approach involved analyzing the genomes of all the microbes in each oral cavity environment they tested.
“Normally when we study a microbial environment, we take samples and only read a small fraction of the genomes present — just enough to ID the broad categories of microbes,” said Meren. “We used a more comprehensive approach called metagenomics, which allowed us to sequence the entire DNA content of our samples from the oral cavity. We were able to reconstruct entire microbial genomes, identifying new microbial species and figuring out where each one fits on the tree of life.”
In one paper (Utter et al.), the researchers studied the microbial residents of three distinct parts of the mouth, providing insight into population structure and spatial arrangement of the oral microbiome and forming hypotheses about adaptation to specific habitats. In the second paper (Shaiber et al.), they focused on one particularly difficult-to-study class of bacteria: Saccharibacteria (TM7). Their results have surprising implications for the evolution of microbes in the mouth.
Different TM7 species could be grouped into six distinct bins, or clades, they found, based the similarities of their genomes, which indicate how recently the different species split from one another in their evolutionary history.
When the team compared those bins to other groups of TM7 species, like those found in the environment outside of the body or in human or animal guts, they were amazed to find that, genetically, instead of the plaque and tongue TM7 species grouping together, the TM7 species from dental plaque grouped more closely with the TM7 species found in soil, while the TM7 species on the tongue more closely resembled those found in the gastrointestinal tract.
“The first time I plotted the phylogeny comparing the TM7 of the tongue and plaque and saw that they were completely separate, my mind exploded,” said first author Alon Shaiber, now a genomics data scientist at Weill Cornell Medicine. “We did not expect that at all.”
 MBL Associate Scientist Jessica Mark Welch in an MBL teaching lab with University of Chicago students. Credit: Ege Yalcindag
MBL Associate Scientist Jessica Mark Welch in an MBL teaching lab with University of Chicago students. Credit: Ege YalcindagThe researchers interpret these results as a hint at how microbes might make the transition from the environment into the human body. “Our hypothesis is that plaque played a role during the evolution of host-associated microbes, such as some clades of TM7, by offering this intermediary space where the bacteria don’t have immediately have to deal with threats from the host,” said Meren. “Once adapted to the plaque, the microbes could then make the jump to adapt to the host entirely, in new habitats like the tongue.
“This was the most exciting thing to us,” he continued. “This shows that the dental plaque, the enemy of our health that we constantly try to get rid of, may at some point have played an important role in the evolution of some of the microbes to call our bodies their home.”
The metagenomics approach meant that the researchers could identify new species of bacteria from the oral cavity that had not previously been studied, due to the challenges of cultivating some of these microbes in the lab.
“The mouth is so easily accessible that people have been working on bacteria from the mouth for a long time,” said Jessica Mark Welch. “But we’re finding that there are entire new microbial groups, including a few really weird and unusual ones, that have not been looked at before.”
Beyond its utility for understanding the evolution and composition of the microbiome, this study and others like it can provide new insights on the role of oral microbes in human health.
“Every environment we look at has these really complicated, complex communities of bacteria, but why is that?” said Mark Welch. “Understanding why these communities are so complex and how the different bacteria interact will help us better understand how to fix a bacterial community that’s damaging our health, telling us which microbes need to be removed or added back in.”
Future research will be aimed at teasing apart the genetic and functional relationships between these newly identified bacterial species, especially in categories of bacteria other than TM7, and how these microbial communities play a role in human biology and disease. The metagenomics approach will also prove useful for studying microbial communities in other places, such as the gut and in environmental settings.
“These kinds of studies are showing us the diversity in the mouth in a new way,” said Mark Welch. “We’re learning about exactly what genes are in different microbes, which will make it possible to model the metabolism of entire communities. The bacteria in the mouth are really a microcosm of ecology, and it relates to the ecology you see at a landscape scale all around us.”
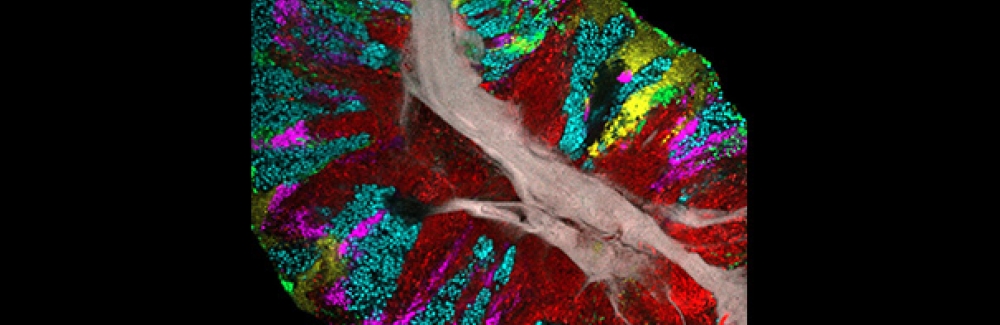
Homepage photo: Bacterial biofilm scraped from the surface of the tongue and imaged using CLASI-FISH. Credit: Steven Wilbert and Gary Borisy, The Forsyth Institute
Citations:
Daniel R. Utter, G. G. Borisy, C.M. Cavanaugh, and J.L. Mark Welch (2020) Metapangenomics of the oral microbiome provides insights into habitat adaptation and cultivar diversity. Genome Biology, DOI: 10.1186/s13059-020-02200-2
Alon Shaiber, A.D. Willis, T.O. Delmont, S. Roux, L-X Chen, A.C. Schmid, M. Yousef, A.R. Watson, K. Lolans, O.C. Esen, S.T.M. Lee, N. Downey, H.G. Morrison, F.E. Dewhirst, J.L. Mark Welch, and A.M Eren (2020) Functional and genetic markers of niche partitioning among enigmatic members of the human oral microbiome. Genome Biology, DOI: 10.1186/s13059-020-02195-w
—###—
The Marine Biological Laboratory (MBL) is dedicated to scientific discovery – exploring fundamental biology, understanding marine biodiversity and the environment, and informing the human condition through research and education. Founded in Woods Hole, Massachusetts in 1888, the MBL is a private, nonprofit institution and an affiliate of the University of Chicago.