A Fresh Spin on Nuclear Centering
WOODS HOLE, Mass. -- Using two specialized microscopes invented at the Marine Biological Laboratory (MBL), a team of researchers from Japan and the MBL have developed a new method to measure the forces that keep the nucleus centered in a living cell. The experiments also provided important new clues about the properties of cellular cytoplasm and the mechanisms of organelle motion within cells. The work was published Oct. 16 in Proceedings of the National Academy of Sciences.
"Understanding the mechanism of nuclear positioning is important in understanding cell division," a central process in early development, growth, and the health of all living organisms, says study lead author Akatsuki Kimura, professor at the Cell Architecture Laboratory at the National Institute of Genetics in Japan. "Cells must divide evenly to produce cells with the same size. For the cell to divide at the center, positioning of the nucleus at the cell center is critical." Kimura adds that "it has been a mystery how a large structure, such as the cell nucleus, can move inside the crowded cell interior."
While it's long been known that appropriate positioning and movement of the nucleus and other organelles are crucial for cell functioning, the ability to accurately measure such intracellular forces has been limited.
Previous work on sea urchin eggs using a magnetic tweezers technique succeeded in measuring forces to move the nucleus, but the underlying mechanism of force production was not clear due to limitations on genetic experimental techniques in this species. The nematode C. elegans, which has a wealth of genetic techniques available, provides another convenient species for investigation of nuclear centering. Rather than the magnetic tweezers method, which implants magnetic beads inside the nucleus that can be moved with an external magnet, the researchers chose a different approach: Spinning the cells, because when a cell is rotated at very high speed, the nucleus is displaced from the center.
The team examined live embryos of C. elegans using two instruments invented at the MBL: the centrifuge polarizing microscope (CPM), developed by the late MBL Distinguished Scientist Shinya Inoué, and the orientation-independent differential interference contrast (OI-DIC) microscope, developed by MBL Senior Scientist Michael Shribak.
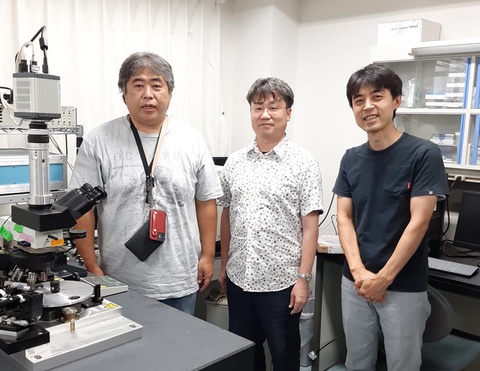
The CPM applies controllable centrifugal forces to a sample by spinning it at high speeds while illuminating it with stroboscopic laser pulses. Using the CPM, Kimura, Makoto Goda (Hamamatsu University School of Medicine), Tomomi Tani (National Institute of Advanced Industrial Science and Technology, then at MBL) and MBL Senior Scientist Rudolf Oldenbourg discovered that when fertilized C. elegans eggs are centrifuged, the cell nucleus is displaced from the center of the cell.
To convert centrifugal speed into force, the research group used the OI-DIC microscope, which characterizes the mass density of the cytoplasm and the nucleus by measuring differences in refractive index, allowing calculation of the precise force acting on the nucleus.
With the CPM and OI-DIC microscopes, "we can now compare the two species [nematode and sea urchin] and discuss the generalities and differences," says Kimura. The work revealed that the force required to move the nucleus in C. elegans was approximately 1/6th less than that measured in the sea urchin, although still larger than theoretically estimated. According to Kimura, "this means that there is an unknown property of the cytoplasm that makes large organelles difficult to move, and which is not accounted for in the current theory."

The nuclear centering mechanism is considered most likely dependent on microtubule activity within the cell, although it's still debated whether microtubules are pushing or pulling against the cell cortex. The results of this study were consistent with the latter mechanism, but further work and perhaps comparison with other research organisms will help settle the question.
"We established a new way to use the power of the CPM and OI-DIC microscopes to measure the force in C. elegans," says Kimura. Because this new technique doesn't require the injection of beads into the cell, like the magnetic or optical tweezers method, it's less complex and more versatile. Now, says Kimura, "we can conduct the experiments in various gene-manipulated cells to reveal the relationship between physical force and genes."
The MBL donated Inoué’s centrifuge polarizing microscope, the only one in existence, to Kimura’s laboratory in 2020.
Citation:
Makoto Goda et al. (2024) Live-cell imaging under centrifugation characterized the cellular force for nuclear centration in the Caenorhabditis elegans embryo. Proc. National Academy of Sciences, DOI: 10.1073/pnas.240275912
—###—
The Marine Biological Laboratory (MBL) is dedicated to scientific discovery – exploring fundamental biology, understanding marine biodiversity and the environment, and informing the human condition through research and education. Founded in Woods Hole, Massachusetts in 1888, the MBL is a private, nonprofit institution and an affiliate of the University of Chicago.